1.
Which of the following statements is not true regarding acids? (3 points)
Acids can be both highly and mildly corrosive.
Most fruits contain weak acids.
Acids react with bases in neutralization reactions to produce water.
Active metals will react with acids in double replacement reactions.
2.
What is not true of a strong base? (3 points)
It must have a concentration above 1.0 M.
It is always a strong electrolyte in water.
It ionizes well in water.
It conducts electricity in solution.
3.
Which of the following solutions will not neutralize a weak base solution of pyridine (C5H5N)? (3 points)
vinegar
ammonia
carbonated soda drink
pickle juice
4.
Which of the following is not an acid-base neutralization reaction? (3 points)
NH3 + HCl 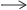 NH4Cl
NH4Cl
H2 + Br2 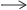 2HBr
2HBr
HCl + HBr 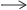 H2 + ClBr
H2 + ClBr
2HBr + Ca(OH)2 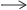 CaBr2 + 2H2O
CaBr2 + 2H2O
5.
What is the salt produced when calcium hydroxide, Ca(OH)2, reacts with phosphoric acid, H3PO4, in a neutralization reaction? (3 points)
CaPO4
Ca3(PO4)2
H2O
Ca2HPO3
6.
Which of the following would decrease the pH level of an acidic solution? (3 points)
adding a base to the acid
increasing the concentration of hydroxide ions
decrease the concentration of hydronium ions
increasing the number of hydrogen ions
7.
Which substance is acting as the Brønsted-Lowry acid in the following chemical reaction? (3 points)
NH4 + OH– 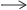 NH3 + H2O
NH3 + H2O
NH4+
OH–
NH3
H2O
8.
If 35.5 mL of 0.23 M HCl is required to completely neutralize 20.0 mL of NH3, what is the concentration of the NH3 solution? Show all of the work needed to solve this problem. (2 points)
HCl + NH3 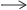 NH4Cl
NH4Cl
9.
What is the pH of a solution with a concentration of 1.3 × 10-3 molar H3O+? Show, or explain, the work used to solve this problem. (2 points)
10.
Explain, in a step by step procedure, how you would use titration to determine the concentration of a calcium hydroxide (Ca(OH)2) solution. (4 points)
11.
A chemical system at equilibrium is referred to as dynamic because the (3 points)
rates of the forward and reverse reactions continue changing.
forward and reverse reactions continue reacting.
value of the equilibrium constant (K) changes.
concentrations of the components continue changing.
12.
If
the forward reaction goes close to completion and has a high yield,
what is the expected value of the equilibrium constant (K)? (2 points)
K = 1
K > 1
K = 0
K < 1
13.
Explain, in terms of particles, concentration, and reaction rate, what you expect to happen when ethene gas (C2H4) and water vapor are sealed in a flask and reach a state of equilibrium. (2 points)
C2H4(g) + H2O(g) 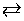 C2H5OH(g)
C2H5OH(g)
1.
Which of the following statements is not true regarding acids? (3 points)
2.
What is not true of a strong base? (3 points)
|
It must have a concentration above 1.0 M. |
|
|
It is always a strong electrolyte in water. |
|
|
It ionizes well in water. |
|
|
It conducts electricity in solution. |
3.
Which of the following solutions will not neutralize a weak base solution of pyridine (C5H5N)? (3 points)
|
vinegar |
|
|
ammonia |
|
|
carbonated soda drink |
|
|
pickle juice |
4.
Which of the following is not an acid-base neutralization reaction? (3 points)
|
NH3 + HCl |
|
|
H2 + Br2 |
|
|
HCl + HBr |
|
|
2HBr + Ca(OH)2 |
5.
What is the salt produced when calcium hydroxide, Ca(OH)2, reacts with phosphoric acid, H3PO4, in a neutralization reaction? (3 points)
|
CaPO4 |
|
|
Ca3(PO4)2 |
|
|
H2O |
|
|
Ca2HPO3 |
6.
Which of the following would decrease the pH level of an acidic solution? (3 points)
|
adding a base to the acid |
|
|
increasing the concentration of hydroxide ions |
|
|
decrease the concentration of hydronium ions |
|
|
increasing the number of hydrogen ions |
7.
Which substance is acting as the Brønsted-Lowry acid in the following chemical reaction? (3 points)
NH4 + OH– 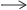 NH3 + H2O
NH3 + H2O
|
NH4+ |
|
|
OH– |
|
|
NH3 |
|
|
H2O |
8.
If 35.5 mL of 0.23 M HCl is required to completely neutralize 20.0 mL of NH3, what is the concentration of the NH3 solution? Show all of the work needed to solve this problem. (2 points)
HCl + NH3  NH4Cl
NH4Cl
9.
What is the pH of a solution with a concentration of 1.3 × 10-3 molar H3O+? Show, or explain, the work used to solve this problem. (2 points)
10.
Explain, in a step by step procedure, how you would use titration to determine the concentration of a calcium hydroxide (Ca(OH)2) solution. (4 points)
11.
A chemical system at equilibrium is referred to as dynamic because the (3 points)
|
rates of the forward and reverse reactions continue changing. |
|
|
forward and reverse reactions continue reacting. |
|
|
value of the equilibrium constant (K) changes. |
|
|
concentrations of the components continue changing. |
12.
If
the forward reaction goes close to completion and has a high yield,
what is the expected value of the equilibrium constant (K)? (2 points)
|
K = 1 |
|
|
K > 1 |
|
|
K = 0 |
|
|
K < 1 |
13.
Explain, in terms of particles, concentration, and reaction rate, what you expect to happen when ethene gas (C2H4) and water vapor are sealed in a flask and reach a state of equilibrium. (2 points)
C2H4(g) + H2O(g)  C2H5OH(g)
C2H5OH(g)
14.
Predict what will happen to the ammonia equilibrium system if hydrogen gas (H2) is added in the following reaction. (2 points)
N2(g) + 3H2(g)  2NH3(g)
2NH3(g)
The equilibrium shifts to the left.
The reaction reaches completion.
The equilibrium remains the same.
The equilibrium shifts to the right.
15.
When considering Le Châtelier’s Principle and the factors that affect chemical equilibrium, which of the following is not true? (2 points)
Increasing the concentration of reactants shifts the equilibrium position to the right.
Decreasing the volume of a system shifts the equilibrium position to the left.
Changing the concentration and volume d
16.
For
the reaction below, describe the temperature and pressure conditions
that would produce the highest yield of the forward reaction. Explain
your answer in terms of Le Châtelier’s principle. (2 points)
N2O4(g)  2NO2(g)
2NO2(g) 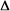 H = +57.2 kJ/mol
H = +57.2 kJ/mol
17.
Which of the following reactions is not an example of an oxidation-reduction reaction? (2 points)
H2 + F2 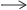 2HF
2HF
Fe + CuSO4 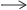 FeSO4 + Cu
FeSO4 + Cu
CuSO4 + 2NaOH 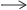 Cu(OH)2 + Na2SO4
Cu(OH)2 + Na2SO4
Cl2 + ZnI2 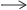 ZnCl2 + I2
ZnCl2 + I2
18.
In the equation below, which of the following is true? (2 points)
2Fe + 6HBr 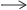 2FeBr3 + 3H2
2FeBr3 + 3H2
H2 is being reduced.
HBr is being oxidized.
Fe is the reducing agent.
FeBr3 is the oxidizing agent.
does not always change the equilibrium constant in a reaction.
Changing the temperature alters both the equilibrium constant and position.
19.
What is the oxidation number of Chlorine in NaClO4? (2 points)
-1
+7
-5
+1
20.
Rusting
is a chemical process that changed the strength and integrity of
objects made of iron or iron alloys. Which of the following statements
correctly describes the rusting process below? (2 points)
4Fe(s) + 3O2(g) 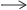 2Fe2O3(s)
2Fe2O3(s)


0 comments